What Does the Future of Mortality Look Like?
By Adam Jedynak and Connie Cheng
Reinsurance News, June 2024

As we emerge from the COVID-19 pandemic, significant questions remain regarding the evolution of mortality in the U.S. This article examines recent shifts in mortality, including residual post-COVID excess mortality and a pre-pandemic slowdown in improvements, particularly within the general population. It explores the impact of medical advancements and societal changes and considers their long-term implications on mortality. For any professional pondering the above question, the insights offered here might shed some light on the path ahead as we navigate the complexities of a post-pandemic world.
Recent General Population Trends During COVID-19
Over time, mortality trends tend to ebb and flow based on a number of factors. Notably, this includes medical advancements, population health and cultural factors, and external factors such as war and pandemics. During the COVID-19 pandemic, mortality rates peaked in winter 2020/21 and winter 2021/22. Since then, deaths have tapered off despite increased case numbers, likely due to widespread vaccinations after 2022, greater accessibility of COVID treatment options, and mildness of subsequent variants. However, some excess mortality from the pandemic is still being observed, with the CDC reporting provisional age-adjusted mortality rates in 2023 at levels about 4.5% higher relative to 2019.
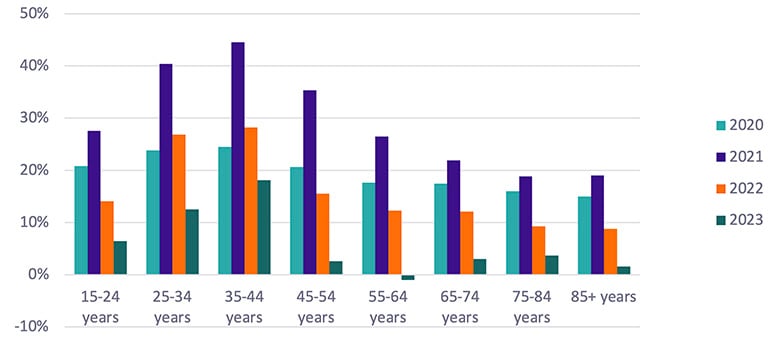
Chart 1 below shows excess mortality as the change in crude death rate per age group against 2019 death rates.
Chart 1
Excess Mortality by Age Group and Year
Source data: CDC
A large disparity is observed by age segment, with elevated mortality still materially present for ages 25–44 as of 2023. This increase in mortality is mainly driven by accidental causes of death, including unintentional injuries and deaths of despair, per CDC data. On the contrary, excess mortality for ages 45+ shows a sharp decline from its peak and is at levels below 5% in 10-year age segments.
As we progress further away from a pre-COVID-19 world, the usage of 2019 (and earlier years) as a precise reference point becomes more tenuous. Chart 1 mentioned previously shows a slightly negative level of excess mortality for ages 55–64, which can still be interpreted as near-zero excess mortality. In general, brief periods of mortality acceleration are observed following large COVID-19 waves for ages 85+, but none that have persisted long enough to materially affect our view of excess mortality overall.
Recent Individual Life-insured Population Trends During COVID-19
Across 2020 through 2022, individual life-insured excess mortality in aggregate was generally in line with or moderately lower than general population levels by age and gender, which are found to be the most meaningful attributes by which to stratify experience. Further, a review of insured population mortality indicates that experience also varied significantly by company. Some companies experienced near-zero excess mortality, while others had sustained excess mortality during only some or all years of the pandemic. Slight variations in excess mortality by face amount bands were also observed, but generally at levels commensurate with the underlying mortality rate differences by face amount band.
However, in 2023, while general population excess mortality dropped to single-digit levels compared to 2019 age-adjusted death rates from the CDC, the same level of decrease was not observed across individual life insurance portfolios. While there was still a downward trend from 2022, high excess mortality continued in older age bands. Excess mortality at ages 25–44 observed in Munich Re Life US data has generally been more muted within the insured population relative to the general population, likely due to the impact of underwriting and differences in socioeconomic covariates.
More recently, provisional monitoring of early 2024 trends suggests that individual life-insured mortality is continuing to trend down closer to general population levels. No sustained impacts of COVID-19 on other causes of death are noted, such as any impacts on the slowdown in mortality improvement for causes of death such as cardiovascular. This is likely because underwriting may help carriers mitigate their exposure to these risks from the onset.
Considerations in Predicting Future Trends
Predicting future mortality trends is a challenging task that occupies not only actuaries but also demographers, medical doctors, epidemiologists, and sociologists. Without a crystal ball, an informed approach grounded in an understanding of historical and current developments is essential to making more accurate predictions. Some important considerations include:
- Historical continuity—Breaking historical improvements into underlying trends (e.g., by cause of death) and underlying drivers (e.g., smoker or obesity prevalence) can provide a basis for future predictions.
- Medical developments—The lengthy process of drug development, from research to mass adoption, offers a preview of future mortality impact, often years in advance.
- Changing drivers—The factors behind most trends (e.g., cancer mortality reduction) often evolve more rapidly than the trends themselves, with future improvements likely driven by new forces.
- Long-term forecasting—The need to understand mortality trends over several decades comes with increasing uncertainty with increasing forecast horizon.
- Unpredictable events—Developments like the COVID-19 pandemic can disrupt trends and introduce another layer of complexity.
Future Mortality Trends
Separating short-term trends (next five to 10 years) and medium- to long-term trends (beyond five to 10 years) provides structure that can also highlight different levels of forecasting confidence.
Short-term Trends
Obesity is associated with higher mortality from cardiovascular conditions, diabetes, and certain cancers. The percentage of the U.S. population classified as obese tripled from 14% in the early 1970s to 42% in 2020.[1] The strongly embedded cultural and economic factors behind this trend, including the popularity of processed foods and a sedentary lifestyle, are likely to fuel the trend going forward.
Cardiovascular conditions—Reducing mortality from cardiovascular conditions such as heart attack and stroke used to be a big part of past mortality improvements. Medical advancements such as statins to control high LDL cholesterol and hypertension medications proved extremely effective. Yet, as the benefit from these breakthrough drugs was realized and new drug pipelines failed to deliver, slowdown in mortality improvements in the past decade does not bode well for the near future.
Opioid crisis—Increases in mortality among younger people due to accidents and opioid overdose in the past decade highlight a real risk of mortality trends not just temporarily slowing down but also reversing. Fentanyl, a now more readily accessible opioid drug that is 50 times more powerful than heroin, is the main culprit as it drastically increases the risk of a fatal overdose. However, a stabilization in opioid deaths in 2023, after a dramatic exponential upward trend, brings back the possibility of mortality improvements for younger individuals.[2]
Post-COVID-19 era—Another important trend to follow relates to the COVID-19 pandemic and remaining excess deaths. This excess should continue to abate over time, but uncertainty around the pace and duration remains.
Other Short-term Developments
The following developments, such as new medical discoveries, have already proven successful, but further impact is expected as new research continues to bring additional breakthroughs and the full benefit of wider adoption materializes.
New obesity drugs—A new generation of drugs, including well-known names like Wegovy and Ozempic, has the potential to accelerate mortality improvements over the coming decades. These drugs mimic the action of the natural hormones, including GLP-1, that regulate appetite, blood sugar levels, and energy expenditure.
Initially developed for diabetes, the drugs have also shown promising effects on weight loss, with average weight loss exceeding 20% for category-leading drugs.[3] Their benefits extend to cardiovascular health, kidney disease, and Parkinson’s, and ongoing trials are assessing their effectiveness against Alzheimer’s, liver disease, sleep apnea, osteoarthritis, and alcohol addiction.
High costs, side effects, and the need for weekly injections present barriers to widespread adoption. Disparities in access could further amplify the gap in mortality improvements by socioeconomic group in coming years. Over time, increased competition and new pill-form versions should reduce costs and side effects, enhancing accessibility.
Liquid biopsy is a cutting-edge technology for early cancer detection through a blood test that identifies cancer biomarkers. Early detection can significantly enhance prognosis and reduce mortality. The GRAIL PATHFINDER 2022 trial detected over twice as many cancers compared to traditional methods, with 71% of these cancers not covered by standard screening tests.[4] Munich Re Life US has entered a multi-year partnership with GRAIL to promote this life-saving technology to U.S. life insurance industry to offer early cancer detection tests to eligible policyholders on a post-issue basis.
Cancer immunotherapy, while not new, is rapidly evolving and showing promising developments. This therapy targets cancer more precisely and is less harmful to the patient's body compared to traditional radiotherapy and chemotherapy. A recent focus in this field involves immune checkpoint inhibitors, which help the body bypass cancer’s ability to evade detection by the immune system.
Medium- to Long-term Trends
Artificial Intelligence (AI)—The recent progress in AI, particularly in the generative AI space, has taken many by surprise and is redefining what is possible. Although most AI breakthroughs have yet to make a meaningful impact on mortality, this technology has major potential to become an accelerator of progress akin to electricity.
The AI Revolution in Medicine book by Peter Lee et. al, highlights areas in health care prone to disruption, from common misdiagnosis and extreme staff shortages due to inefficiencies from administrative and insurance-related work. New large language models have already been shown to outperform medical doctors in diagnosing some rare and complex cases.
However, the initial impact of AI is likely to come from enhancing rather than replacing human professionals, providing support with administration, sourcing information, and automating selected tasks. The benefit will come from freeing up doctors and allowing them to spend more time with patients. Additionally, AI solutions like Amazon HealthLake promise to convert the vast amount of health care data from unstructured to structured format, enhancing diagnosis, treatment, and medical studies.
Progress around precision medicine, which promises treatments tailored to individuals’ needs, has been hindered by high costs and the need for large datasets. AI and big data provide the automation needed to make this technology feasible. This technology can enable better disease classification, recognition of differences between patients, and the creation of personalized treatment plans.
Drug discovery and development, traditionally a lengthy process with many failures and few successes, consumes vast resources. The scale and complexity make it a prime candidate for disruption. AI assists by analyzing hundreds of potential candidate molecules, matching candidates with clinical trials, and analyzing and documenting results. AlphaFold, released by Alphabet, is an algorithm for predicting the structure of proteins and was used to create a full database of 200 million proteins. Previous efforts using traditional techniques took decades and only allowed 170,000 proteins to be mapped. This breakthrough has profound implications for disease understanding and drug discovery.[5]
Autonomous vehicles—Each year, around 40,000 people die in car accidents in the U.S. alone.[6] This scary statistic is hard to comprehend as it is equivalent to two passenger planes crashing every single week. Ninety-four percent of all car accidents are attributed to human error.[7]
Self-driving cars offer a promise to prevent many of these fatalities. Although the challenge is great, notable advancements have been made, with Tesla and Alphabet's Waymo vehicles already autonomously navigating U.S. roads. That said, the technology is not perfect just yet, and further enhancements are essential to achieve the safety standards necessary for broader regulatory approval. Both technology refinement and approvals are crucial for widespread adoption and a meaningful reduction in mortality.
Gene therapy and CRISPR-Cas9 enable targeted and precise DNA editing, enhancing cancer immunotherapy by modifying T-cells to better recognize and attack cancer cells. This technology also holds promise for genetic and hereditary diseases (e.g., sickle cell disease) and disease prevention by utilizing natural immunity shown in some individuals. Additionally, it supports drug development by enabling more precise biological models.
mRNA vaccines replace the real virus used in traditional vaccines with synthetic mRNA code. This approach results in rapid development, scalable production, and precise targeting as first demonstrated with COVID-19 vaccines. New application areas include flu, cancer prevention, and reducing the risk of cancer relapse. For instance, a new Moderna mRNA vaccine has been shown to reduce melanoma recurrence or deaths by 49% in patients who recovered from stage III/IV melanoma.[8]
In a discussion on the future of cancer treatments, Dr. Brad Heltemes, a medical director at Munich Re, highlighted the promising advances in the field: "The potential to stimulate and guide the immune system to target cancer cells more precisely is substantial. Utilizing our body's natural defense mechanisms allows for both a safer and more comprehensive treatment approach, enabling us to find hidden nests of cancer cells that are not apparent with current techniques. With developments across multiple fronts, including mRNA vaccines, targeted monoclonal antibodies, and adoptive T-cell therapies—along with the possibility of combining these approaches—there is significant optimism for reducing cancer mortality. However, as is common with most new technologies, substantial costs are involved, which could limit availability and slow the progress being made."
Targeting aging directly, organ replacement, and regenerative medicine might sound like science fiction to many. Still the question remains: Can you ignore this distant and low-likelihood possibility considering the profound implications it can bring to the insurance industry?
Limited past successes justify skepticism. Past progress has been confined to the cellular level (stem cell research) and less complex biological organisms. Nevertheless, certain recent breakthroughs provide hope. For example, a preclinical study by scientists from Duke-NUS Medical School demonstrated that blocking a protein called interleukin-11 allows kidney cells to regenerate and restore kidney function.[9] The current research focus is unlikely to impact mortality soon, but it is still an important driver for better understanding the aging process, setting foundations for potentially unfathomable breakthroughs in the distant future.
In conclusion, the future of mortality in the post-COVID-19 era presents a complex interplay of factors, including the lingering effects of the pandemic, advancements in medical technology, and societal changes. While we face ongoing challenges such as obesity and the opioid crisis, innovative developments in drug therapies and technology offer hope for significant improvements in long-term mortality rates. Furthermore, preparedness for black swan events like pandemics underscores the importance of vigilance and adaptability in health policy and medical practice to navigate the evolving landscape of mortality trends effectively.
Statements of fact and opinions expressed herein are those of the individual authors and are not necessarily those of the Society of Actuaries, the newsletter editors, or the respective authors’ employers.
Adam Jedynak, FIA, MBA, is a director & actuary for Munich Re Company Canada Branch (Life). Adam can be contacted at ajedynak@munichre.ca.
Connie Cheng, FSA, MAAA, is an AVP & actuary for Munich Re Life US. Connie can be contacted at ccheng@munichre.com.
Endnotes
[1]https://www.soa.org/EPiServer/Cms/#context=epi.cms.contentdata:///93616&viewsetting=viewlanguage:///en US obesity rates have tripled oveears, Ur the last 60 ySA Facts, March 21, 2023; Adult Obesity Facts, U.S. Centers for Disease Control and Prevention, May 17, 2022.
[2] US drug overdose deaths top 109,000 in the past year, Reuters, June 14, 2023; Drug Overdose Deaths in the United States, 2002–2022, U.S. Centers for Disease Control and Prevention, March 2024.
[3] For example, Tirzepatide Once Weekly for the Treatment of Obesity, New England Journal of Medicine, June 4, 2022.
[4] GRAIL Announces Final Results From the PATHFINDER Multi-Cancer Early Detection Screening Study at ESMO Congress 2022, Sept. 11, 2022.
[5] DeepMind has predicted the structure of almost every protein known to science, MIT Technology Review, July 28, 2022
[6] NHTSA Estimates for 2022 Show Roadway Fatalities Remain Flat After Two Years of Dramatic Increases, U.S. Department of Transportation, April 20, 2023; Preliminary Semiannual Estimates, NSC Injury Facts.
[7] USDOT Releases 2016 Fatal Traffic Crash Data, U.S. Department of Transportation, October 6, 2017.
[8] Moderna And Merck Announce mRNA-4157 (V940) In Combination with Keytruda(R) (Pembrolizumab) Demonstrated Continued Improvement in Recurrence-Free Survival and Distant Metastasis-Free Survival in Patients with High-Risk Stage III/IV Melanoma Following Complete Resection Versus Keytruda at Three Years, Dec. 14, 2023.
[9] Widjaja, A.A., Viswanathan, S., Shekeran, S.G. et al. Targeting endogenous kidney regeneration using anti-IL11 therapy in acute and chronic models of kidney disease. Nat Commun 13, 7497 (2022).