Biosimilars, Part 5: Payer Decision Making
By Gregory Warren and Tony Pistilli
Health Watch, January 2024

This article has been edited and updated since its original publication on the Axene Health Partners website.
Parts 1 and 2 of this article provided an introduction to the field of biosimilars and an overview of how their growth is changing the pharmaceutical market, respectively. Parts 3 and 4 examined the effects of biosimilar launches for one of the most widely used brand-name drugs and the financial impact such launches may have. In this issue, we finish our series with an analysis of how payers will decide which, if any, biosimilars to cover and how biosimilar adoption may be maximized.
Once the first Humira biosimilar, Amjevita, launched in the United States in January of 2023, payers needed to begin making decisions about coverage, not only for Amjevita, but for all the other Humira biosimilars launched in 2023: on or off formulary, coverage tier, prior authorizations and so on. A continuum of payer responses might include anything from doing nothing (stay with just Humira) to adding selected biosimilars to the formulary (how many—a few? many? all?) to the most aggressive approach of removing Humira from the formulary entirely.
Payer Priorities
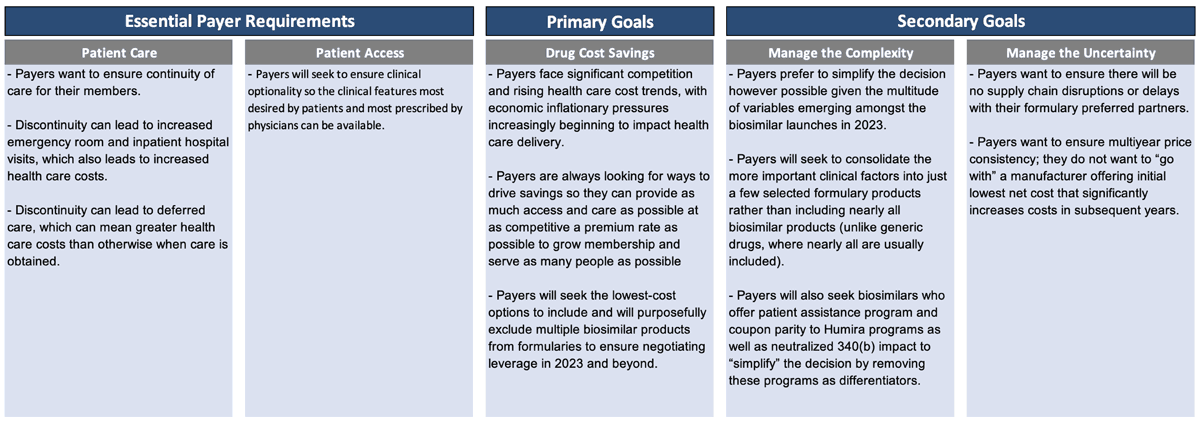
So, how do payers think about this? What are their priorities? What is their decision-making process? We believe payers generally have some essential requirements, one primary goal and a couple secondary goals (Figure 1).
Figure 1
Payer Priorities for Biosimilar Decision Making
Essential payer requirements include making sure patients get access to the care they truly need. Discontinuity of care can lead to increased health care costs in the short term and over time. Payers want patients to have access that provides clinical optionality so the right clinical features are available.
Once that foundation is established, then the primary goal is really cost savings. Payers face significant competition and rising health care cost trends, with economic inflationary pressures beginning to impact health care delivery.
Payers are constantly looking for ways to drive savings so they can provide as much access and care as possible at as competitive a premium rate as possible. Then they can grow membership and serve as many people as possible. Payers seek to include the lowest-cost options and purposefully exclude multiple products from the formulary to create the negotiating leverage to accomplish that.
Once they identify how to accomplish their primary goal, payers will then pursue secondary goals. Payers want to manage the complexity and uncertainty in this market, so they want to simplify the decision, which is difficult to accomplish with all the Humira biosimilars that launched in 2023.
Managing Complexity and Uncertainty
A lot of differences exist among the Humira biosimilars, which is unusual in a generics market, once again demonstrating that biosimilars are not generics. The many differences increase payers’ interest in simplifying the complexity.
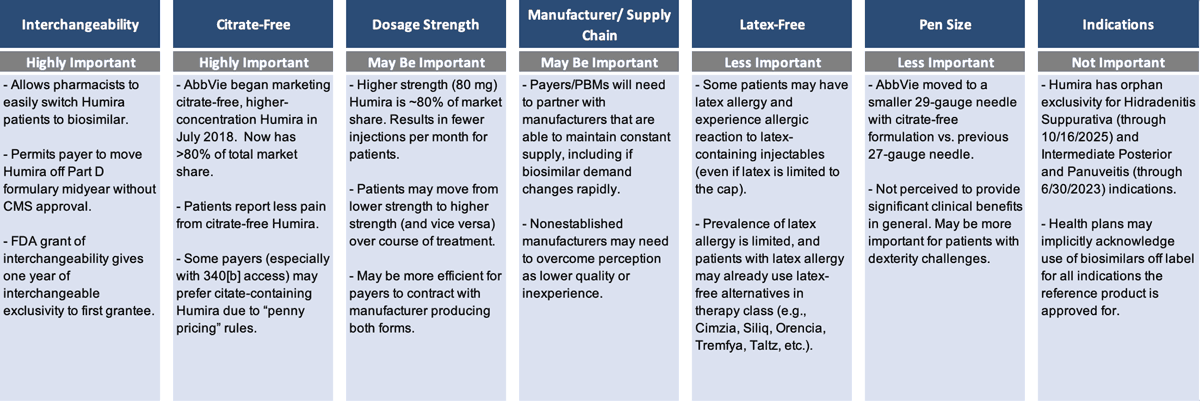
Payers want to be confident that whichever manufacturers are preferred on their formulary have the infrastructure and supply chain to ensure there are no disruptions or delays. They want to make sure there is going to be multiyear price consistency. And they want to simplify the complexity of the various clinical factors that differentiate the Humira biosimilars (Figure 2).
Figure 2
Key Clinical Factors Differentiating Humira Biosimilars
Among the different clinical factors, having interchangeability and being citrate-free both seem to be highly important; dosage strength may be important; and being latex-free and having a specific pen size are less important. Obviously, the less important factors are very important for the patients they impact, but they’re less important when you're looking at a population strategy as a whole.
Manufacturer Pricing Strategies
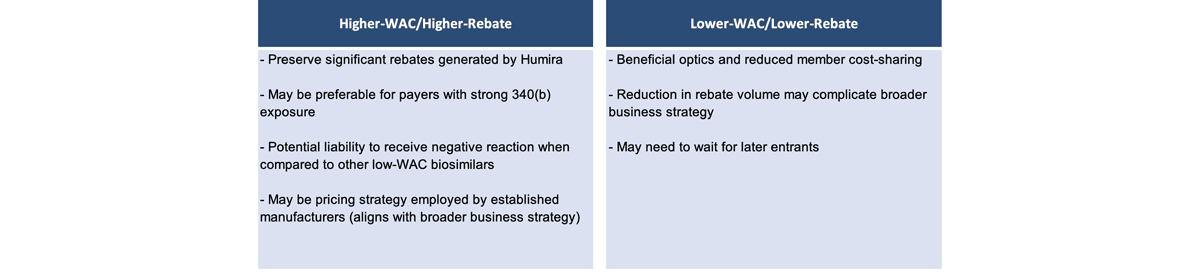
Manufacturer pricing will likely bifurcate into two main strategies (Figure 3). The first, with a higher wholesale acquisition cost (WAC) and higher rebate, preserves significant rebates that are generated by Humira and may be preferable for payers with strong 340(b) exposure. However, it may be a liability and receive negative reactions when compared with other lower-WAC biosimilars. There may be pressure in the media and widespread public opinion to push toward a second strategy with a lower WAC price, because then there is a lower ingredient cost, a lower allowed cost amount and lower cost-sharing for members.
Figure 3
Two Main Strategies of Manufacturer Pricing
Cost-Saving Considerations by Book of Business
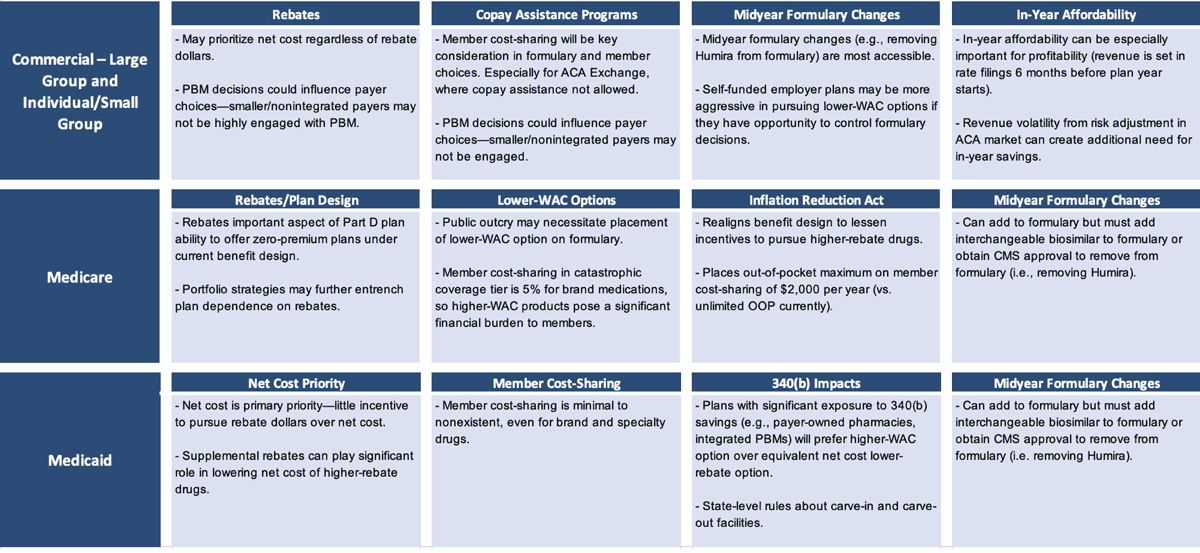
Cost-saving considerations differ by book of business (Figure 4). If a lower-WAC product is put on the formulary (even more so if it is put on a preferred tier), then a pharmacy benefit manager (PBM) with aggressive client rebate guarantees that don’t include a lot of exception language could be at risk of missing those guarantees.
Those employers and health plans that have a tightly worded rebate guarantee in their PBM contract could now see that work against them (or against their patients), in a sense, because their PBM may not be incentivized to include or prefer a lower-WAC option on their formulary. Humira may contribute more than one-third of total plan rebate dollars. It could further entrench itself by increasing rebate percentages as various biosimilars launch. PBMs may have an incentive to delay the adoption of biosimilars if those products do not offer total rebate dollars similar to those from Humira.
Figure 4
Cost Considerations by Book of Business
In Medicare, rebates have a leveraging effect that incentivizes higher rebates more than lower-WAC prices in that the higher rebates are advantageous in driving lower premium rates that are more competitive to enrolling members. The Inflation Reduction Act reduces that reliance on rebates to some extent, but the reliance is still there and still substantial. In Medicaid plans, patients typically have pretty low cost-sharing. Thus, the goal of trying to mitigate member cost-sharing with a lower-WAC strategy is less relevant in the Medicaid book of business.
Regulatory Impediments to Making Immediate Formulary Changes
Timing matters. Medicare actuaries knew that Medicare bids for 2024 coverage were due early in June 2023. At that point, only Humira and Amjevita were approved on the market. None of the others launched until July. Price negotiations are not supposed to occur until approval to launch. Commercial Employer and ACA Exchange markets have different timing flexibilities.
So, even though 2024 Medicare formularies did not do anything dramatic, like kicking Humira off formulary, they still took the opportunity to add some other biosimilars to their formularies midyear. They would not have been able to remove Humira until an interchangeable product was approved, and even then 60 days’ notice was required. But that would have been a very dramatic step to take.
Summary
We will be watching what happens in 2024 and the years beyond regarding potentially more active movement with Humira. It will be interesting to see which manufacturers are reaching their market share goals heading into 2024 and which are not. That could define which manufacturers take dramatic steps in price declines or merger and acquisition activity if they are not hitting their expectations. We may be close to entering the wildest curl in this coming wave of biosimilars. Buckle up!
Statements of fact and opinions expressed herein are those of the individual authors and are not necessarily those of the Society of Actuaries, the editors, or the respective authors’ employers.
Gregory Warren, FSA, MAAA, FCA, is a partner and consulting actuary at Axene Health Partners. Greg can be reached at greg.warren@axenehp.com.
Tony Pistilli, FSA, MAAA, FCA, CERA, CPC, is a consulting actuary at Axene Health Partners. Tony can be reached at tony.pistilli@axenehp.com.